ISO 17100 - MDR & IVDR are on the way

Safety advantages of ISO 17100 - current and future
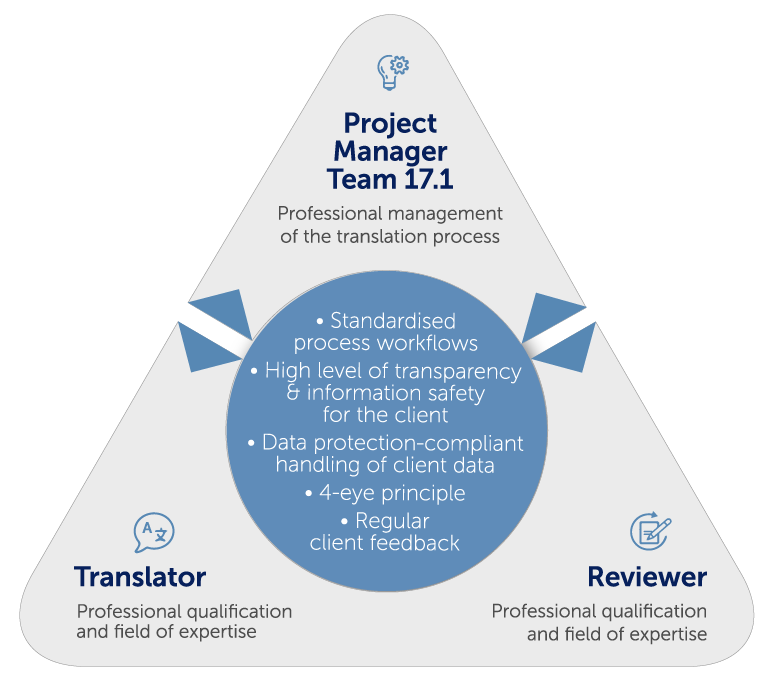
mt-g stands for impeccable medical and pharmaceutical translations. We achieve this through clearly defined processes across the entire translation chain. An essential cornerstone of our quality management is the international standard DIN EN ISO 9001.
Structuring a translation according to DIN EN ISO 17100 increases the quality through even higher qualification and technical competence requirements and gives you additional security compared to the conventional translation process – and not only in terms of the upcoming MDR and IVDR regulations. The worldwide standard also has a positive effect on your audits, as you benefit from verifiable and recognised procedures.
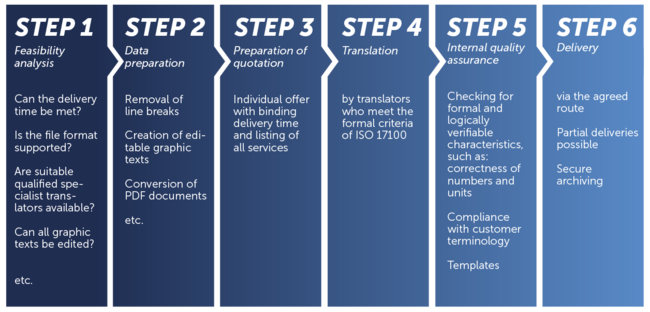
Standard translation process according to ISO 9001
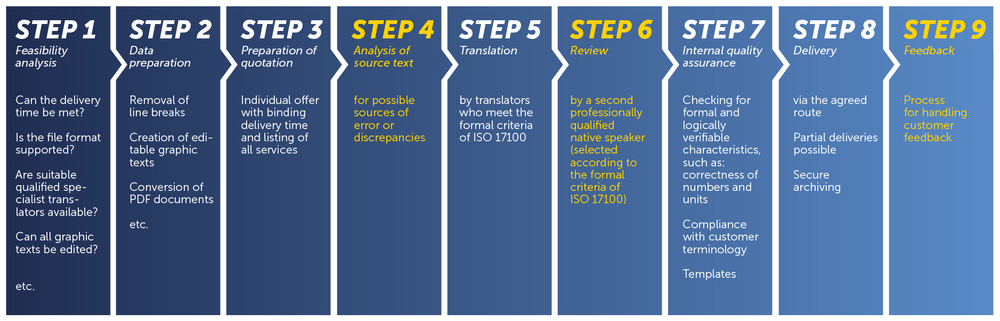
Translation process according to ISO 17100
Translators & Reviewers
- hold a degree in translation, linguistics or language studies or an equivalent degree that includes
significant translation training, from a recognized institution of higher education, or - a degree in any other field from a recognized institution of higher education and has the equivalent of two years of full-time professional experience in translating, or
- has the equivalent of five years of full-time professional experience in translating
Project Managers
- have expertise in organising and supporting the translation process
- participate in continuous training
As of May 26, 2021, the new MDR (Medical Device Regulation 2017/745) will apply. On May 26, 2022, the In-Vitro Diagnostics Regulation (IVDR) of the European Union will become effective. These regulations will tighten many requirements for the manufacturers of medical devices, including those regarding translation and localisation: translations must be “correct and state-of-the-art” (Art. 16 MDR and Art. 16 IVDR).
Consequently, the comprehensibility and accuracy of translations – in up to 24 languages – will become increasingly important. Translation processes according to ISO 17100 give you, your customers and your Notified Bodies the assurance of receiving compliant translations.
We understand that you, as a manufacturer of medical products, are responsible for the health and safety of your customers, which is why translations of package inserts, labels or web texts are to be classified as product components. You can expect the greatest possible care from us when providing these translations.
We work exclusively with expert translators who incorporate customer-specific terminology, your company’s wording as well as relevant specifications into their translations and save them in translation databases, known as Translation Memory Systems, securely stored for you by mt-g. This helps to ensure that technical terms are then reused in future translations. Wording and terminology are continually refined through long-term cooperation, thus reducing translation costs.